 Available for Import
Available for Import
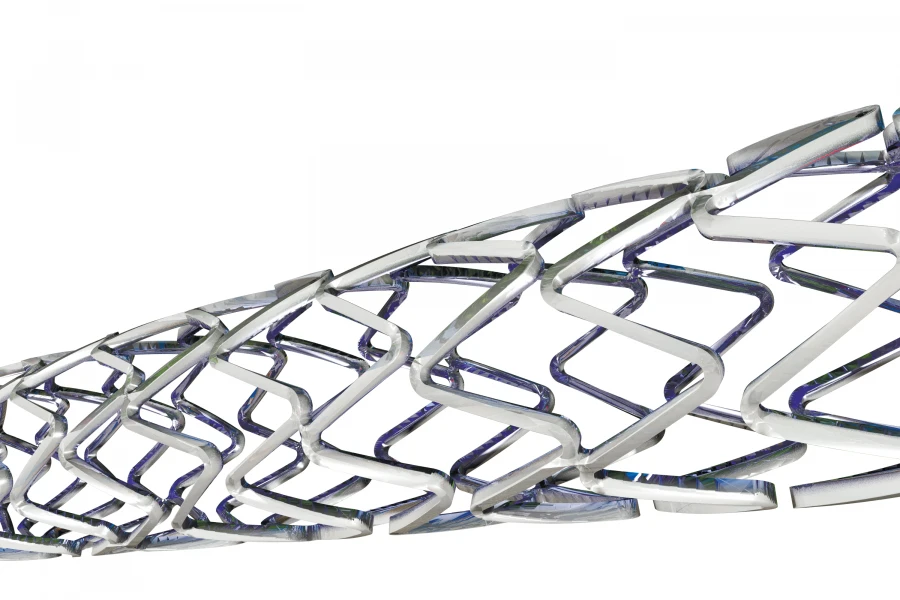
Non-Coated Metal Coronary Artery Stent for Maintaining Vessel Patency
Bulk pricing available
FOB, CIF & EXW terms available
Description
A sterile non-absorbable tubular device [uncoated metallic stent] intended for implantation into a coronary artery or saphenous vein graft of the heart to maintain cavitary patency of the vessel and increase the lumen diameter in patients with symptomatic atherosclerotic heart disease. The product is guided to the implantation site under radiographic control using a balloon catheter that causes the product to expand when the balloon is inflated. It is made of cobalt-chromium alloy (Co-Cr). It presents a mesh structure.
Specifications
Share your requirements for a quick response!
Delivery & Payment
Shipping Terms
Delivery Time
Payment Methods
Similar Products You May Be Interested In
Straight Polypectomy Forceps, Model TSH-04-041-24.5, 245 mm
View Details
D1 Type Cytobrush for Biological Material Collection
View Details
Intramedullary Bone Screw, Article 530.001
View Details
Sterile Single-Use Urogenital Polymer Catheter Type A (Universal)
View Details
Hip Joint Total Prosthesis Pin Extractor Tool, 103.011
View Details
Disposable Sterile Polymer Vaginal Speculum Type 3 Size S
View Details
Randall Stone Removal Forceps No. 4 - 31-107-22
View Details
Distal Hole Blocking Tool for Intramedullary Osteosynthesis, Art. 520.353
View Details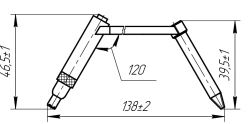
Combined Guide for Self-Tapping Screw, Code 306.518
View Details
Bone Punch Tool for Plate Blade Preparation, Model 29.26.12
View Details
Intramedullary Control Probes, 136mm, Art. 500.548
View Details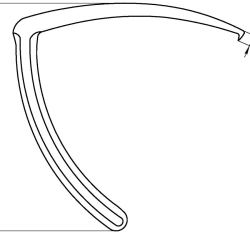
Channel Formation Tool, Model OM.008.02.00
View DetailsVerified Suppliers
All products are sourced directly from authorized Russian manufacturers
Quality Assurance
Products meet international quality standards with proper certification
Global Shipping
Reliable logistics solutions to deliver products to your location
Secure Payments
Multiple secure payment options to facilitate international transactions