 Available for Import
Available for Import
Non-Sterile Implantable Plates 1/3 Tubular 3.5 for Fracture Fixation of Fibula and Ankle
Bulk pricing available
FOB, CIF & EXW terms available
Description
Non-sterile implantable plate made of solid material intended for osteosynthesis of fractures of the fibula and ankle. Localisation of the plate is diaphyseal and distal part of the bone. Shape - straight, rounded on the cut along the radius R5.
Universal design for right and left limbs. The holes for screw insertion without angular stability are groove-shaped. The edges of the plate are rounded around the perimeter.
Laser marking of the product article. Must be sterilised before use.
Specifications
Share your requirements for a quick response!
Delivery & Payment
Shipping Terms
Delivery Time
Payment Methods
Similar Products You May Be Interested In

Antegrade-Retrograde Cannulated Femoral Rod Ø11, Length 300-440mm, Step 20mm, Code 200.11-XXX
View Details
Sponge Screw with Cylindrical Thread for Bone Stabilization
View Details
Intramedullary Femoral Pins Ø 12 mm, Length 285 mm, Code 627.285
View Details
Non-Sterile Titanium Blocking Screw, 6.5 mm Diameter, 70-120 mm Length, Step 5 mm, Model 26.24.XXX
View Details
Cortical Screw 5mm Diameter (Length 20-80mm)
View Details
Cortical Screw for Bone Fixation and Osteosynthesis
View Details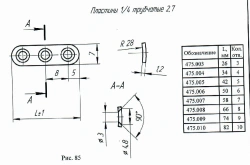
Non-Sterile Implant Plate 1/4 Tubular 2.7, Art. 475.005
View Details
Distal Locking Screw for Bone Fractures
View Details
Carrying Rod for External Fixation (Diameter 9mm-12mm, Length 150-400mm)
View Details
Tapered Pin with Four Longitudinal Grooves
View Details
Medical Transport Immobilization Splint Set KSHI-01-Medplant
View Details
Titanium Bone Repositioning Pins, 4mm/2.5mm, Art. 481.080
View DetailsVerified Suppliers
All products are sourced directly from authorized Russian manufacturers
Quality Assurance
Products meet international quality standards with proper certification
Global Shipping
Reliable logistics solutions to deliver products to your location
Secure Payments
Multiple secure payment options to facilitate international transactions