 Available for Import
Available for Import
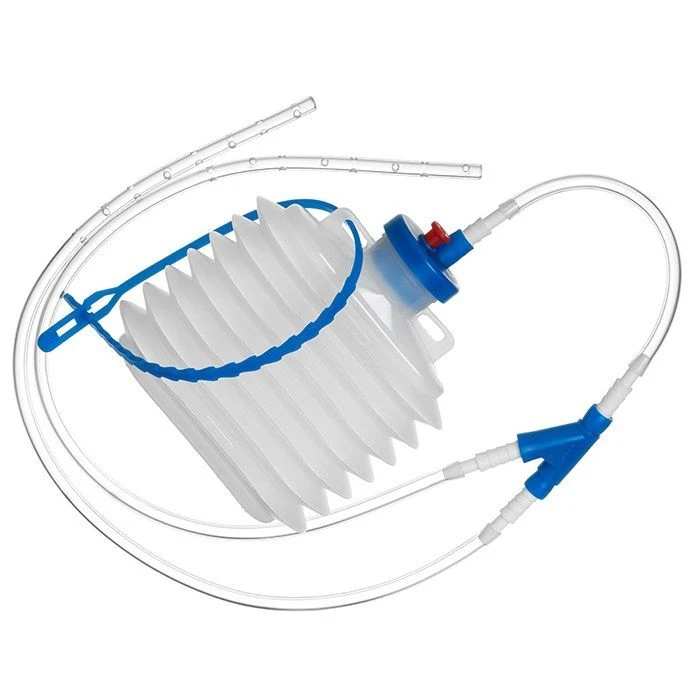
Sterile Single-Use Active Wound Drainage Device with Balloon for Effective Wound Care
Bulk pricing available
FOB, CIF & EXW terms available
Description
The device for active drainage of wounds of single application is intended for active (due to vacuum) drainage of wounds in pre- or postoperative periods. It is made of polymeric materials for medical use. The device consists of a corrugated balloon of 250 ml and a drainage unit with two tubes of 380 mm length and 5.5 mm external diameter.
The corrugated balloon is fitted with a removable stopper. On the inner side of the cork of the cylinder there is a non-return valve, which prevents accidental ingress of biological fluid back into the drainage tube and ensures the closure of the drainage system from contact with the external environment when the tube is disconnected from the cylinder. An air valve is located on the exterior of the cylinder stopper, which provides convenience for personnel to operate the device.
Also on the outer side of the cork there is a connector to which the drainage unit is connected through an adapter. On the cylinder there is a fixing element.
Specifications
Share your requirements for a quick response!
Delivery & Payment
Shipping Terms
Delivery Time
Payment Methods
Similar Products You May Be Interested In

Adult Tongue Holder - VZ-Y-4
View Details
Capillary Blood Collection System "Sintavet 200" - Sintavet-200 Fluoride Lithium with K3EDTA Filler
View Details
Surgical Needle Holder, Multi-Surface, 250mm, VZ-I-10-3
View Details
7 mm Combination Wrench for Osteosynthesis, Article 390.087
View Details
Medical Extension Tube Single Use 6.0x1500 mm with Female Connectors
View Details
Elastic Pediatric Bowel Clamp, Straight, 170 mm, TZ-01-330-17
View Details
Disposable Polymer Gynecological Mirror KUSKO No. 1 (S) with Central Rotating Lock
View Details
Intramedullary Osteosynthesis Guide, Article 535.100
View Details
Disposable Sterile Polymer Gynecological Mirror 2-Part Model №3 (L)
View Details
UNIVAC® Vacuum Blood Collection Tube with Silica Gel Activator 13x75mm
View Details
Left Guide Attachment for Intramedullary Osteosynthesis, Article 500.610
View Details
Curved Guide for Spinal Surgery, Article 855.005
View DetailsVerified Suppliers
All products are sourced directly from authorized Russian manufacturers
Quality Assurance
Products meet international quality standards with proper certification
Global Shipping
Reliable logistics solutions to deliver products to your location
Secure Payments
Multiple secure payment options to facilitate international transactions